At Precisionary Instruments, because so many of our tissue slicer users handle fixed tissue, we often get asked about antigen retrieval. Today, we’d like to cover this as a short explanatory topic!
What is antigen retrieval and why is it important?
Have you ever worked with fixed tissue? If you process tissue slices for immunological purposes, then it’s likely that you have! Methods like immunohistochemistry help stain specific proteins. But when proteins undergo fixation with chemicals like formaldehyde, amino acids can undergo undesired cross-linking. This cross-linking may mask tissue antigens and prevent antibodies from binding to your protein of choice. As a result, you will not be able to detect the protein you want to see on immunohistochemistry or in-situ hybridization. Here is a quick diagram that explains this process visually (Figure 1):
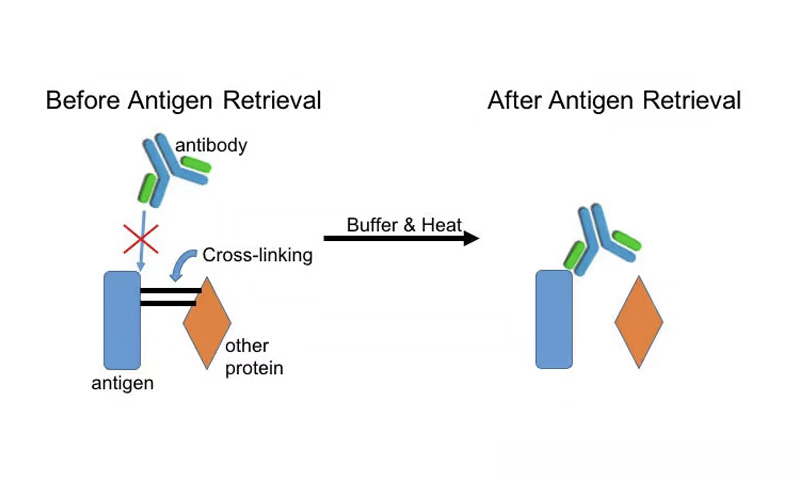
Antigen retrieval is the method to help unmask antigenic epitopes that may be covered by cross-linked proteins. This process uses buffers and heat to break cross-links, or methylene bridges, between proteins, allowing the antigenic site to be exposed again for antibody binding. Therefore, antigen retrieval is a method completed before immunohistochemistry or in-situ hybridization staining, in order to maximize your signal.
What techniques are used for antigen retrieval?
Antigen retrieval techniques need to be optimized because each person may want to target a different antigen, use a different antibody, study different tissue types, and/or employ different methods of fixation. There are two main ways to recover epitope sites:
- Heat-induced epitope retrieval (HIER)
- Proteolytic-induced epitope retrieval (PIER)
HIER is the most common method for antigen retrieval. It also tends to be more successful than PIER. If you use cryostats or rotary microtomes to cut fixed tissue for pathology, then HIER is the preferred method. In this method, heat (like a microwave oven, water bath, etc) is used to reverse some cross-links and restore epitope structure.
PIER focuses on the “proteolytic” capabilities of enzymes like proteinase K, trypsin, and pepsin to digest cross-links. However, these may have broad proteolytic activity, so other protein sites like the antigen. Thus, there is the risk of PIER destroying tissue morphology and that’s why it has a lower success rate compared to HIER.
For more information about immunological staining, check out this publication.