Explore our detailed manual for mastering PCLS in respiratory research
We’re thrilled to announce the launch of our comprehensive Precision-Cut Lung Slices Protocol Manual—a practical, researcher-friendly guide designed to help you get consistent, healthy lung tissue slices every time. Whether you’re just getting started with PCLS or are looking for ways to refine your technique, this guide walks you through every step of the process, from sample collection to long-term culture.
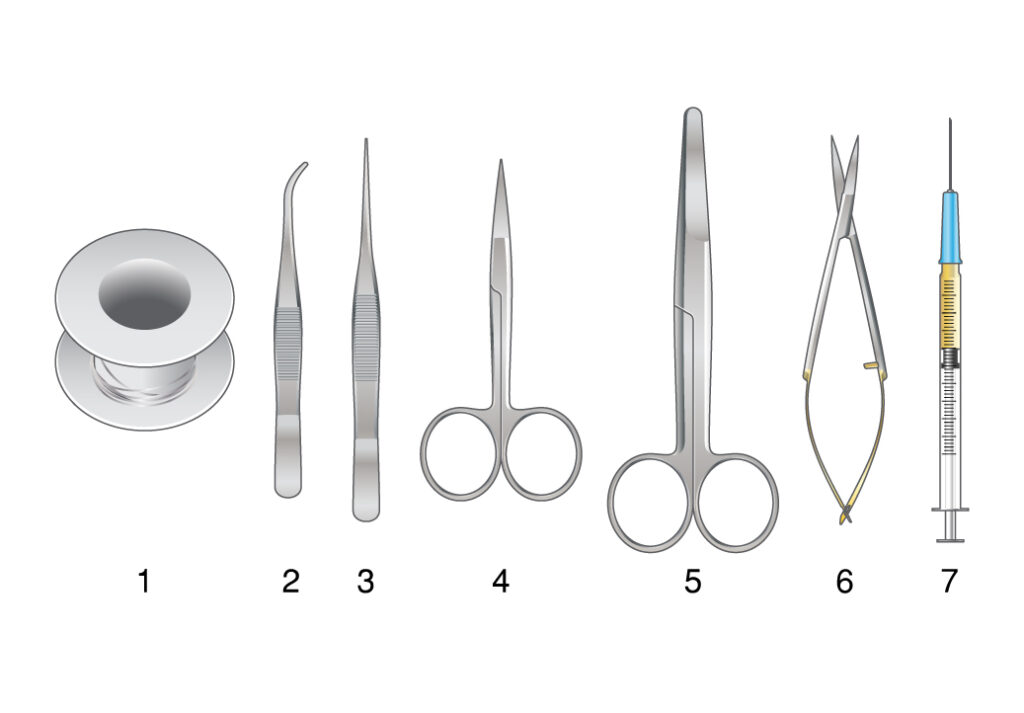
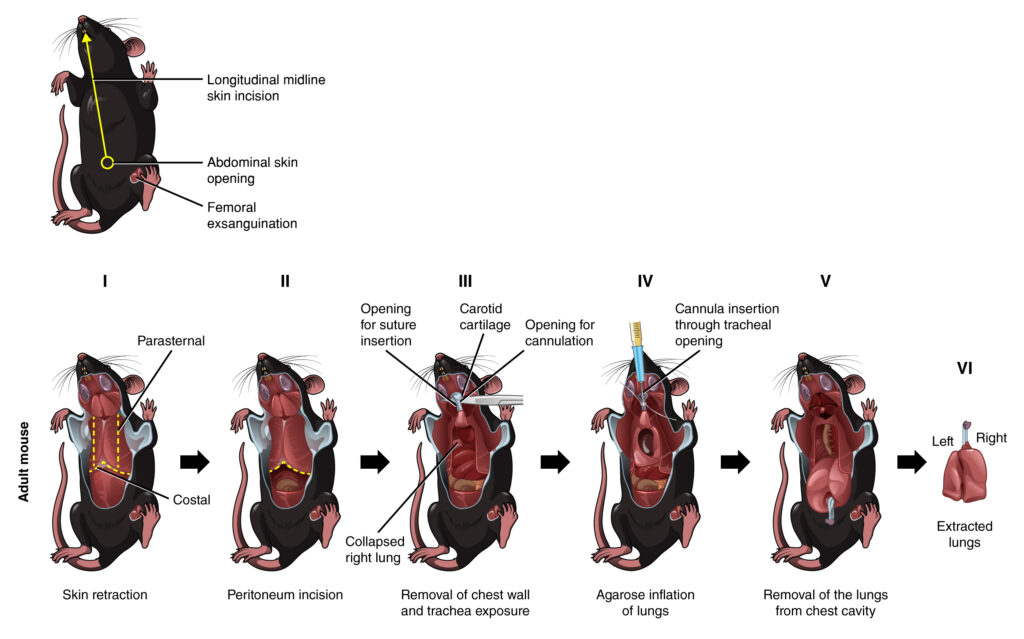
Figure 3. Comprehensive workflow for mouse dissection and lung harvesting.
Dissection equipment includes suture, curved forceps, fine tip forceps, fine tip dissecting scissors, surgical scissors, spring scissors, and pentobarbital in a 1 ml syringe attached with a hypodermic needle. The skin incision line is depicted for reference. The process involves two main scenarios: C. Adult mouse dissection, agarose inflation, and harvesting of lungs; and D. Postnatal day 3 (P3) mouse dissection, agarose inflation, and lung harvesting?
Why Precision-Cut Lung Slices?
In lung research, having a reliable ex vivo model is key. That’s where Precision-Cut Lung Slices come in. Unlike 2D cell cultures or even organoids, PCLS preserve the structural and cellular complexity of lung tissue—including intact alveoli—making them ideal for studying everything from injury and inflammation to regeneration and repair.
The manual explains why PCLS are a powerful bridge between traditional in vitro methods and whole-animal models. Plus, they support the 3Rs by reducing animal use: one lung can yield dozens of slices for parallel experiments.
What’s Inside the Manual?
- A detailed overview of PCLS applications
- Step-by-step protocol using the Compresstome vibratome for smooth, even slices
- Troubleshooting tips for optimizing slice viability and minimizing tissue damage
- Best practices for embedding, sectioning, recovering, and culturing mouse lung slices
- Special insights on real-time imaging and alveolar development models
From new grad students to senior investigators, this guide is built to support anyone involved in respiratory research or lung-focused therapeutic testing.
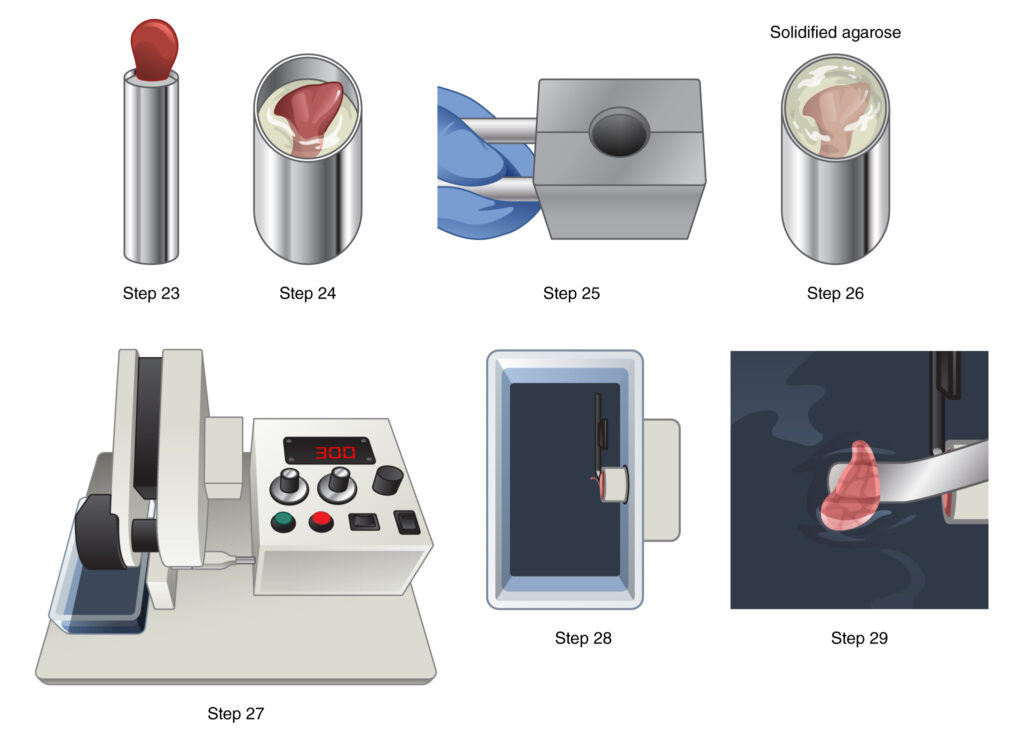
Figure 4. Setup for precision-cut lung slicing and live ima-ging. A. The lung slicer utilized is the Vibratome, specifically the Compresstome® VF-300-OZ model. B. A syringe chilling block is employed for solidifying agarose. C. Agarose-inflated lungs harvested from a P3 mouse are shown. D. A separated left lung lobe is placed on a laboratory tissue roll. E. A schematic diagram illustrates agarose embedding of the left lung lobe on a specimen holder for making precision-cut lung slices (PCLS). F. Preci-sion-cut lung slices are arranged in a 24-well plate. G. Transwell modification facilitates the stabilization of lung slices on the well plate. H. Precision-cut lung slices are set in an ibidi 24-well u-pla-te using modified transwells and metal flat washers (black rim). A schematic demonstrates the relative position and alignment of the metal flat washer, transwell, lung slice, and 40x long working distance air objective lens on an inverted microscope for live imaging.
Who Will Find This Useful?
This protocol is for:
- Scientists starting their first PCLS experiments\n
- Experienced users who want to improve consistency and troubleshooting\n
- Research mentors training students or technicians\n
- Labs transitioning from animal-based workflows to ex vivo lung models
Download the full PCLS Protocol Manual now to start building or refining your lung slice workflow.
Questions or custom experimental needs? Reach out to our team—we’re happy to support your research with one-on-one guidance.
[Download the Protocol] | [Contact Our Scientific Team]