Getting Started with Cardiac Slicing
Welcome, fellow scientists, to a journey into the fascinating realm of cardiac tissue slicing! In the spirit of Valentine’s Day, where hearts take center stage as symbols of love and affection, let’s explore the intricate world of cardiac tissue and how precision slicing can unveil its mysteries.
The History of Cardiac Slicing
Our journey through cardiac tissue slicing begins with a brief historical overview. This technique has been evolving for over eight decades, with some groundbreaking advancements in recent years. Pioneers like Pincus and Thienes laid the foundation, but it was in 1992 when Parrish and colleagues introduced thin heart slices that the potential of this technology truly shone. These slices allowed for extended culture while maintaining viability and metabolism. Fast forward a decade, and Brandenburger and team simplified the process further by introducing a method using transwell membranes. These developments highlight the evolving significance of cardiac sectioning in advancing our understanding of the heart.
Why Cardiac Slices Matter
Now, let’s delve into why cardiac slices are so important in the world of cardiovascular research. In translational cardiovascular research, accurate, standardized, long-term, and rapid drug testing is crucial. Conventional myocardial culture systems often fall short, but cardiac slices offer a solution. These thin slices of adult ventricular tissue maintain the adult phenotype for extended periods within a multi-cell environment. They provide a cost-effective means to quantitatively characterize drug-related changes at the cellular level, covering factors like contractility, protein expression, transcriptional profiles, calcium homeostasis, and electrophysiology. In short, cardiac slices are a game-changer in advancing cardiovascular research.
In the visual representation below, we explore the diverse platforms used to model cardiac physiology in culture. These platforms encompass adult cardiomyocytes, hiPS-CMs, Ventricular wedges, and heart slices. Furthermore, recent advancements have taken heart slice technology to new heights. Several research groups have introduced innovative approaches, such as heart slices on-a-chip, incorporating preload, including preload/afterload, and enhancing the culture medium with essential nutrients. These developments have expanded the possibilities for modeling and understanding cardiac physiology in culture, ushering in exciting new avenues for research.

Making Live Cardiac Slices
Creating live cardiac slices is no easy task and requires meticulous attention to detail. Factors like blade speed, blade deflection, and cutting plane alignment play a crucial role. Thin heart slices, a relatively recent technology, have opened up new possibilities. These slices maintain the adult phenotype, offer high viability, and preserve typical tissue characteristics for up to 24 hours. Plus, they maintain a three-dimensional structure within a multi-cell environment, allowing for essential interactions between cellular components and the extracellular matrix.
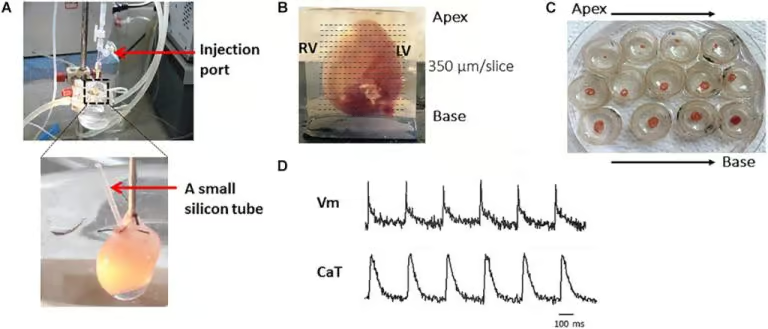
In the figure above, we highlight the key steps of the protocol for studying mouse heart tissue. These steps involve obtaining representative mouse heart and transverse slices, from apex to base, to facilitate the research process.
- Langendoff Perfusion: The protocol begins with Langendoff perfusion steps, represented in panel A.
- Embedding and Slicing: Following perfusion, the heart is embedded in 4% low-melting agarose, as in panel B, and then precisely cut at a right angle to its long axis using a vibratome.
- Transfer to Petri Dishes: The resulting slices are carefully transferred to petri dishes, as illustrated in panel C.
- Recording Voltage and Calcium Transients: The final step involves recording voltage and calcium transients in the prepared heart slices, as shown in panel D.
Choosing the Right Equipment and Buffer Solutions
In the world of cardiac slice preparation, choosing the right tools and buffer solutions is essential. The Compresstome vibratome stands out as the top choice for precision and reliability in creating cardiac slices with utmost accuracy. Maintaining the right environment for tissue preservation is equally important. Submerging the tissue in specialized buffer solutions, like ice-cold oxygenated BDM-containing HEPES-buffered solution or blebbistatin-containing bicarbonate-buffered solution, ensures tissue integrity and viability throughout the preparation process.
The Importance of Cutting Orientation
To achieve optimal cardiac slice preparation, consider cutting the ventricular tissue tangentially to the epicardial surface. This technique aligns slices with the local cell orientation and maintains tissue stiffness. A high-precision vibratome equipped with a ceramic blade is crucial for accurate sectioning along the epicardium-tangential plane.
Recovery Time and Temperature Effects
Recovery time after cutting is essential for cardiac slices to attain electrophysiological equilibrium. Different species may have varying recovery times, typically around 35 minutes for guinea pigs and approximately 60 minutes for rabbits. The use of ice-cold solution during preparation prevents ischemic conditions in the tissue block but can lead to transient alterations in electrophysiological measurements.
Slice Thickness Matters
Finally, consider the thickness of your cardiac slices. Researchers tend to opt for slices not exceeding 400 μm to ensure adequate oxygen diffusion reaches all cells within the section, minimizing the risk of hypoxic conditions.
Key Takeaways for Cardiac Slicing
In conclusion, cardiac tissue slicing is a fascinating and vital technique in cardiovascular research. It offers a window into the complexities of the heart and contributes significantly to our understanding of cardiac function. Here are the key takeaways for making cardiac slices for research:
- Cut tissue in an epicardium-tangential plane with a high-precision vibratome and aim for slices no thicker than 400 μm.
- Allow slices prepared in ice-cold solution a recovery period in a warm solution to attain steady-state electrophysiological properties. Recovery time may vary by species, typically around 35 minutes for guinea pigs and approximately 60 minutes for rabbits.
Here is a short video that takes you through the entire process of embedding and sectioning a mouse heart using a Compresstome vibratome. This video was made by Professor James Smyth, PhD, from Virginia Tech: