Introducing Professor Claudia Loebel and Her Team
At the University of Pennsylvania’s Department of Bioengineering and the Center for Precision Engineering for Health (CPE4H), Professor Claudia Loebel leads a dynamic lab that’s redefining how engineering can impact human health. After relocating from the University of Michigan, her lab has settled into a thriving research environment with cutting-edge facilities.
Her team, which includes four PhD students, three postdoctoral fellows, and a research assistant (Figure 1), comes from a wide range of backgrounds, including materials science, bioengineering, and cancer biology. Together, they’re united by a shared mission: to develop biomaterials and technologies that improve our understanding of how cells interact with their surrounding environments, particularly in the context of tissue repair and regeneration.

Focusing on the Science Behind Tissue Repair
Professor Loebel’s lab is deeply invested in exploring how tissues regenerate and respond to damage, especially within the pulmonary system. Using both in vitro and in vivo models, her work dives into the extracellular matrix—an often-overlooked component that plays a vital role in how cells behave and function.
A standout area of research involves mimicking early-stage fibrosis, a condition where tissue becomes abnormally stiff. By replicating these changes in the lab, her team is gaining insights into disease progression and opening the door to potential therapeutic approaches.
How the Compresstome Vibratome Plays a Role
The Compresstome vibratome is an indispensable tool in Professor Loebel’s lab, enabling precise preparation of precision-cut lung slices (PCLS). These tissue slices are vital for ex vivo culture, where researchers can study lung behavior in controlled conditions.
One particularly innovative technique developed in the lab involves in-situ crosslinking, which allows them to stiffen the extracellular matrix within tissue slices. This method, based on dityrosine bond formation, helps replicate the early stages of fibrosis in a highly controlled manner (Figure 2). Importantly, this approach isn’t limited to lung tissue—it’s also being used with slices from the skin, liver, and heart.
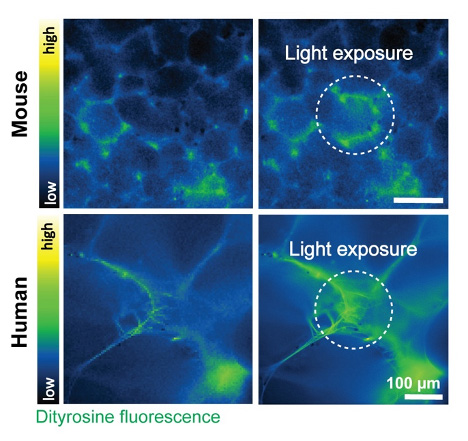
The precision of the Compresstome ensures consistent results, which is essential when exploring how subtle changes in tissue properties affect cell behavior. This work has already made a significant impact, including a recent research publication on bioRxiv that highlights how locally stiffened tissue matrices regulate cellular function.
Looking Ahead for PCLS in Respiratory Research
Professor Loebel’s work represents an exciting intersection of bioengineering and biology, with tangible implications for human health. By leveraging tools like the Compresstome vibratome, her lab is paving the way for new discoveries in tissue engineering and regenerative medicine.
Through her team’s dedication and innovative approaches, Professor Loebel is not only advancing our understanding of complex diseases like fibrosis but also equipping the broader scientific community with valuable insights and techniques. It’s inspiring to see how a focus on precision and collaboration can drive meaningful progress.